Myelodysplastic syndrome (MDS) is a clonal hematopoietic disorder characterized by cytopenias and an increased risk of progression to acute myeloid leukemia (AML). Its pathogenesis is strictly linked to chromosomal instability, which in turn provides a valuable prognostic marker. Malignant cells develop alternative routes to escape mitosis checkpoints, overcoming the mitotic arrest imposed by Spindle Assembly Checkpoint (SAC), a process dependent on CDC20 inactivation. Abnormal levels of CDC20 can inhibit mitotic arrest, promoting premature exit from mitosis. Overexpression of CEP55 also facilitates the mitotic exit, resulting in polyploidy (an event called Mitotic Slippage). Since chromosomal abnormalities are one of the most important prognostic factors for patients with MDS, this study aimed to analyze the possible link between chromosomal abnormalities and CDC20 and CEP55 mRNA expression in MDS.
We evaluated the bone marrow cells from 45 patients diagnosed as MDS according to 2016 WHO-classification (1 MDS-SLD, 15 MDS-RS-MLD, 5 MDS-MLD, 1 t-MDS, and 23 MDS-EB) and 5 bone marrow of healthy controls. Conventional Karyotyping was performed by G-banding of 20 metaphases whenever possible. TaqMan expression assays for CDC20 (Hs00426680_mH) and CEP55 (Hs01070181_m1) were performed in duplicate and the expression ratios were calculated using the 2−ΔCq method. Normality was evaluated by Shapiro-Wilk test. Outliers were removed. The Student's t-test or one-way ANOVA with Tukey/Games Howell post-hoc test was used to analyze the influence of relative expression regarding variables.
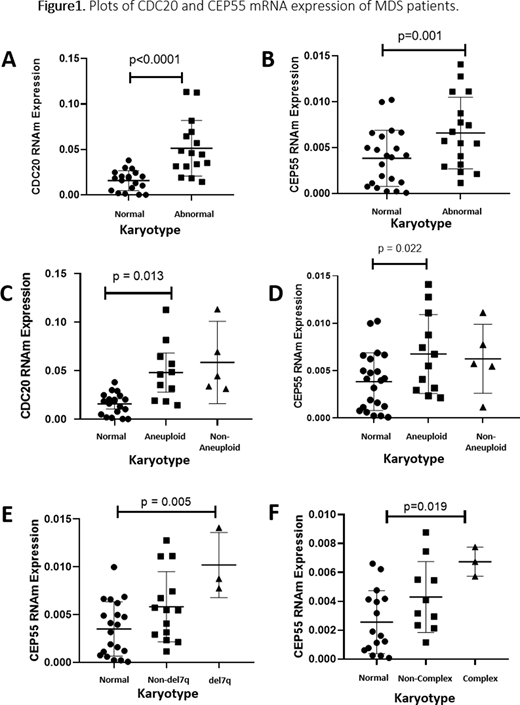
Patients with MDS showed increased expression of CDC20 and CEP55 compared to healthy individuals (p<0.0001 and p<0.0001). Regarding karyotype, there was the overexpression of CDC20 and CEP55 in patients with altered karyotype and aneuploid karyotype when compared to patients with normal karyotype (p <0.0001 and p =0.001; p = 0.013 and p = 0.022, respectively) (Figure 1A-D). CDC20 and CEP55 have fundamental functions in controlling the progression of metaphase to anaphase and both, when upregulated, induce chromosomal instability. Additionally, patients with del(7q) and complex karyotype showed hyperexpression of CEP55 when compared with patients with normal karyotype (p = 0.005 and p = 0.019) (Figure 1E-F), while patients with deletion (5q) had an increased expression of CDC20 when compared with patients with normal karyotype (p <0.0001). Our group previously demonstrate that high CDC20 protein expression is associated with complex karyotype in MDS patients. Thus, we hypothesized that the deregulation of CDC20 and CEP55 expression induces chromosomal changes, each one in its way. Both can cause disturbances in crucial phases of mitosis (anaphase and cytokinesis, respectively). Finally, we detected a strong correlation between CDC20 and CEP55 (r = 0.646; p <0.0001), suggesting both genes may play a synergistic role during chromosomal abnormalities in MDS, creating possible new targets to be evaluated in MDS.
Our data suggest CDC20 and CEP55 as possible new therapeutic targets in MDS. There is a need for further studies, validations and urgent in-depth investigations in cell lines/primary samples or murine models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal